1. Fuel Cells
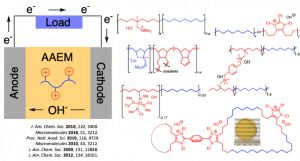
Fuel cells are electrochemical devices that efficiently produce energy, offering advantages such as low emissions and high power density. Currently, most commercially available systems employ proton exchange membranes (PEMs) as electrolytes and operate under acidic conditions. However, these systems suffer from sluggish reduction kinetics and require costly platinum as an electrode material, which limits widespread use of PEM-based fuel cells. To bypass the obstacles associated with PEMs, research interest has recently focused on alkaline fuel cells (AFCs) that utilize anion exchange membranes (AEMs). This approach avoids the use of precious metals and would make devices more affordable, but the development of efficient alkaline AEMs remains a major challenge.
To date, our group has designed polyelectrolyte membranes for AFCs that exhibit competitive properties with other materials in the field. We take advantage of the functional group tolerance of Grubbs’ 2nd generation catalyst for ring-opening metathesis polymerizations (ROMP) of functionalized monomers. This approach provides well-defined polymers containing cationic moieties without the need for post- polymerization modification. At the same time, our group has explored small molecule cationic moieties and developed a family of imidazolium based cations that do not degrade even under the harshest basic conditions. These cations are promising for incorporation into polymers to prepare AEMs with superior base stability.
-
Hugar, K.; You, W.; Coates, G. W. ACS Energy Lett., 2019, 4, 1681–1686. doi: 10.1021/acsenergylett.9b00908
-
You, W.; Noonan, K. J. T.; Coates, G. W. Prog. Polym. Sci., 2020, 100, 101177. doi: 10.1016/j.progpolymsci.2019.101177
-
You, W.; Hugar, K. M.; Selhorst, R. C.; Treichel, M.; Peltier, C. R.; Noonan, K. J. T.; Coates, G. W. J. Org. Chem., 2020, 86, 254−263. doi: 10.1021/acs.joc.0c02051
-
You, W.; Ganley, J. M.; Ernst, B. G.; Peltier, C. R.; Ko, H.; DiStasio J.r., R. A.; Knowles, R. R.; Coates, G. W. Chem. Sci., 2021. doi:10.1039/D0SC05789D
2. Batteries
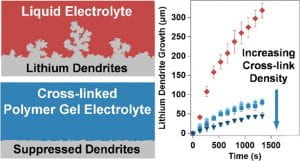
The development of lithium ion batteries (LIBs) has revolutionized portable devices and electric vehicles. Currently, commercial LIBs utilize organic solvents as the electrolyte. However, these flammable liquids raise great safety concerns if the battery is accidently ruptured or if internal thermal runaway happens. Polymer electrolytes are viewed as safer alternatives to liquid electrolytes. However, the most widely used polymer electrolyte – polyethylene oxide (PEO) –suffers from low conductivity at ambient temperature. Increasing the room temperature conductivities of polymer electrolytes has attracted the interest of many researchers. Additionally, next generation high energy lithium batteries require pure lithium metal as the anode material. Lithium metal has been known to have a problem with dendrite growth, which shortens the lifetime of the battery. Various methods have been evaluated for Li dendrite suppression but this problem remains largely unsolved.
Our group has designed a family of cross-linked hydrocarbon/poly(ethylene oxide) polymer electrolytes that not only have high room temperature conductivity but also suppress Li dendrites significantly. We further elucidated how the dendrite growth is influenced by macromolecular composition. This new family of polymer electrolytes is promising for use in lithium metal batteries. We also prepared polymer electrolytes with polyesters synthesized in our group and explored how lithium ions are transported in these polymers with the help of computational chemists. This collaboration gives us insights into how polymer structure affects the lithium conduction and opens up a significant new design space for polymer electrolytes. We have also investigated organic cathode materials.
Selected References:
- Khurana, R.; Schaefer, J. L.; Archer, L. A.; Coates, G. W. J. Am. Chem. Soc. 2014, 136, 7395–7402. doi:10.1021/ja502133j
-
Sanjuna, S.; Tikekar, M.; Biswal, P.; Li, G.; Johnson, H. E.N.; Deng, Y.; Zhao, Q.; Vu, D.; Coates, G. W.; Archer, L. A. Nano Lett., 2020, 20, 5749−5758. doi: 10.1021/acs.nanolett.0c01501
-
Sanjuna, S.; Johnson, H. E.N.; Biswal, P.; Vu, D.; Zhao, Q.; Yin, J.; Abel, B. A.; Deng, Y.; Coates, G. W.; Archer, L. A. Macromolecule, 2020, 13 , 5445−5454. doi: 10.1021/acs.macromol.0c00475
-
Gao, K. W.; Loo, W. S.; Snyder, R.L.; Abel, Brooks A.; Choo, Y.; Lee, A.; Teixeira, S. C. M.; Garetz, B. A.; Coates, G. W.; Balsara, N. P. Macromolecules, 2020, 14, 5728−5739. doi: 10.1021/acs.macromol.0c00747
-
Snyder, R.L.; Choo, Y.; Gao, K. W.; Halat, D. M.; Abel, B. A.; Sundararaman, S.; Prendergast, D.; Reimer, J.A.;Balsara, N. P.; Coates, G. W. ACS Energy Lett., 2021. doi: 10.1021/acsenergylett.1c00594